Deep learning for accurate diagnosis of liver tumor based on magnetic resonance imaging and clinical data
Background
Early-stage diagnosis and treatment can improve survival rates of liver cancer patients. Dynamic contrast-enhanced MRI provides the most comprehensive information for differential diagnosis of liver tumors. However, MRI diagnosis is affected by subjective experience, so deep learning may supply a new diagnostic strategy. We used convolutional neural networks (CNNs) to develop a deep learning system (DLS) to classify liver tumors based on enhanced MR images, unenhanced MR images, and clinical data including text and laboratory test results.
Methods
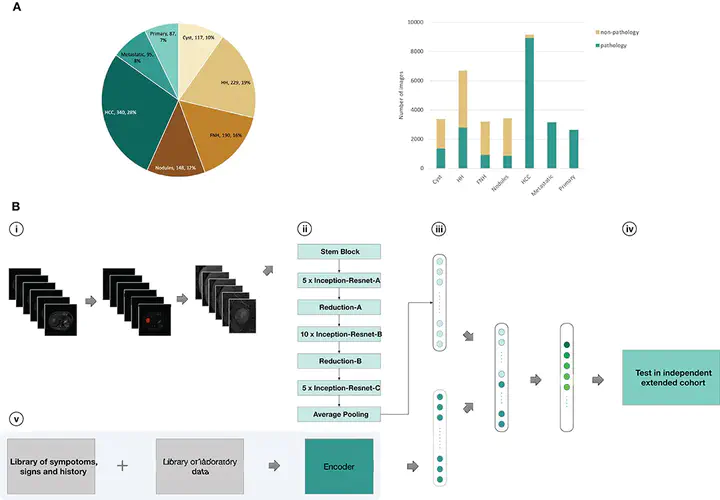
Using data from 1,210 patients with liver tumors (N = 31,608 images), we trained CNNs to get seven-way classifiers, binary classifiers, and three-way malignancy-classifiers (Model A-Model G). Models were validated in an external independent extended cohort of 201 patients (N = 6,816 images). The area under receiver operating characteristic (ROC) curve (AUC) were compared across different models. We also compared the sensitivity and specificity of models with the performance of three experienced radiologists.
Results
Deep learning achieves a performance on par with three experienced radiologists on classifying liver tumors in seven categories. Using only unenhanced images, CNN performs well in distinguishing malignant from benign liver tumors (AUC, 0.946; 95% CI 0.914–0.979 vs. 0.951; 0.919–0.982, P = 0.664). New CNN combining unenhanced images with clinical data greatly improved the performance of classifying malignancies as hepatocellular carcinoma (AUC, 0.985; 95% CI 0.960–1.000), metastatic tumors (0.998; 0.989–1.000), and other primary malignancies (0.963; 0.896–1.000), and the agreement with pathology was 91.9%.These models mined diagnostic information in unenhanced images and clinical data by deep-neural-network, which were different to previous methods that utilized enhanced images. The sensitivity and specificity of almost every category in these models reached the same high level compared to three experienced radiologists.
Conclusion
Trained with data in various acquisition conditions, DLS that integrated these models could be used as an accurate and time-saving assisted-diagnostic strategy for liver tumors in clinical settings, even in the absence of contrast agents. DLS therefore has the potential to avoid contrast-related side effects and reduce economic costs associated with current standard MRI inspection practices for liver tumor patients.